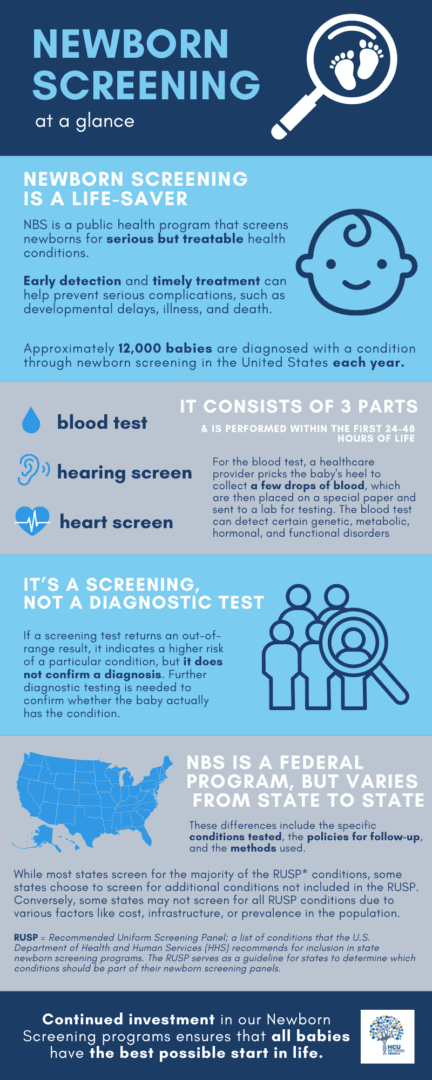
Newborn screening identifies conditions that can affect a child’s long-term health or survival. Early detection, diagnosis, and intervention can prevent death or disability and enable children to reach their full potential. Each year, millions of babies in the U.S. are routinely screened, using a few drops of blood from the newborn’s heel, for certain genetic, endocrine, and metabolic disorders, and are also tested for hearing loss and critical congenital heart defects (CCHDs) prior to discharge from a hospital or birthing center. (Source: Newborn Screening Portal | CDC)
Classical Homocystinuria (HCU) is an inborn error of the metabolism that affects an estimated 1 in 200,000 newborns in the U.S., although that number may be much higher since the current newborn screening protocols used in the U.S. do not identify all HCU newborns.
Up to 50% of babies born with Classical Homocystinuria (HCU) are being missed by the newborn screening protocols currently used by most U.S. states. These babies are usually diagnosed years later after symptoms develop, and this delay can have serious health consequences.
Although HCU officially became part of the Recommended Uniform Screening Panel in 2009, issues with screening protocols continue to result in missed cases. See below how current NBS protocols are impacting HCU diagnoses:
- The biomarker used to screen for HCU, Methionine (MET), is not as sensitive as another biomarker called total plasma homocysteine (tHCY) – yet many newborns are only being tested for MET and not tHCY.
- The window of time when the screening blood specimen is collected - within 24–48 hours after birth – is too soon to detect HCU in many affected newborns, who typically do not have positive bloodwork for or show signs of increased MET until after this period.
U.S. and international experts in newborn screening for HCU have recommended a revised process that includes a lower cut-off for MET and/or a corresponding ratio of MET to Phenylalanine (PHE). The lower cutoff for MET can range from 39 to 50 µmol/L, depending on lab median. Additionally, experts recommend a second-tier test for tHCY using the same dried blood spot.
This revision can positively impact remethylation disorders as well. The two-tier process described above can help identify patients with severe MTHFR and specific cobalamin disorders, if the algorithm also includes a low cut-off for MET and MET/PHE ratio on the first screen. For those that fall under the cut-off, a second-tier screen for tHCY is recommended. While severe MTHFR and cobalamin disorders are not primary conditions on RUSP, they are serious disorders and patients can benefit from early diagnosis. It is estimated that 1:100,000 to 150,000 babies are born each year in the U.S. with these disorders.
The algorithm may also utilize a lower cutoff for propionyl carnitine (C3-AC) and a second-tier test for methylcitric acid (MCA) and methylmalonic acid (MMA); this may improve diagnosis of propionate disorders. The CDC’s first-tier HCY assay would also capture these patients with much more accuracy.
CDC's HCU Screening Efforts
The CDC's Newborn Screening and Molecular Biology Branch also developed and validated a novel assay that multiplexes homocysteine in a first-tier newborn screening method along with other biomarkers. Homocysteine is more specific for screening homocystinuria (HCU) than the currently used biomarker, methionine. This novel assay can significantly improve HCU detection in newborns.
Models of How States Can Revise and Have Revised Screening - Case Studies from NY, MA, and CO Newborn Screening Labs.
- HCU NBS Infographic
- HCU Fact Sheet
- Newborn Screening Stakeholder Letter
- Webinar: Classical HCU: A Journey to Improve Outcomes Through NBS (2022)
- Patient Stories
- Timeline of When States Adopted HCU Screening - Were you or your child missed by newborn screening? Please review the table below before to see when newborn screening was implemented in your state. If you or your child were missed after that date, please share our newborn screening survey with your clinic!
You don't fit the criteria?
That's okay - still forward the survey to your metabolic team to make sure they are aware of the survey!
| AK | 2003 |
| AL | 2004 |
| AR | 2008 |
| AZ | 1979 |
| CA | 2005 |
| CO | 2006 |
| CT | 1993 |
| DC | 1996 |
| DE | 1979-1999, 2003 |
| FL | 2006 |
| GA | 1978 |
| HI | 2002 |
| IA | 2003 |
| ID | 2002 |
| IL | 2002 |
| IN | 1985 |
| KS | 2008 |
| KY | 2006 |
| LA | 2004 |
| MA | 1968 |
| MD | |
| ME | |
| MI | 2004 |
| MN | 2001 |
| MO | |
| MS | |
| MT | |
| NC | |
| ND | 2003 |
| NE | 2008 |
| NH | |
| NJ | |
| NM | |
| NV | |
| NY | |
| OH | |
| OK | 2008 |
| OR | 2003 |
| PA | |
| RI | |
| SC | |
| SD | |
| TN | 2004 |
| TX | 2006 |
| UT | 2006 |
| VA | 1984 |
| VT | |
| WA | 2004 |
| WI | 2003 |
| WV | |
| WY | 2006 |
For more information, please contact HCU Network America’s Executive Director, Danae Bartke, at dbartke@hcunetworkamerica.org
