Your dollars at work:
Updates from the 2022 CblC research grant!
March 2023
Silvia Vilasi (Institute of Biophysics (IBF), National Research Council (CNR), Italy) Principal Investigator of the project “Identification of Compounds to Rescue MMACHC Functional Deficiency in CBLC Disease” granted by HCU Network America, Organic Acidemia Association and CblC Onlus Italian Association, tells us what objectives have been achieved during the first phases of the project and in which direction the research activities are going.
The long-term goal of our project is to find a novel pharmacological strategy to ameliorate CblC symptoms, exploiting a multidisciplinary approach that blends physics, chemistry and biology. To this end, we also need to better understand the impact of pathological mutations on the structure and function of MMACHC protein.
For these reasons during these first six months, we focused on the characterization of two pathological variants, selected together with Prof Carlo Dionisi Vici, Head of Clinical and Research Unit of Metabolic Diseases at the Ospedale Pediatrico Bambino Gesù, Rome (Italy) as representative of patients population. In parallel, we also started the screening of small molecules, with drug-like characteristics, able to bind MMACHC protein and hopefully revert the pathogenic phenotype.
By using molecular biology techniques, we paved the way for research by obtaining the isolated MMACHC protein, both in its functional form, the so-called wild-type protein, and two pathological variants resulting from the mutations identified in CblC patients. Then, we tried to answer some key questions: what is the structure of the mutated proteins, and how does it differ from the wild-type, fully functional one? How do these proteins arrange themselves in the space? Are these mutant proteins able to maintain the correct shape when subjected to external stressors, i.e. are the mutated forms less stable than the wild type one? Can the mutated proteins still bind to another copy of itself (i.e. form a dimer) as observed for the functional protein? Are the mutated proteins able to bind vitamin B12, and promote its conversion?
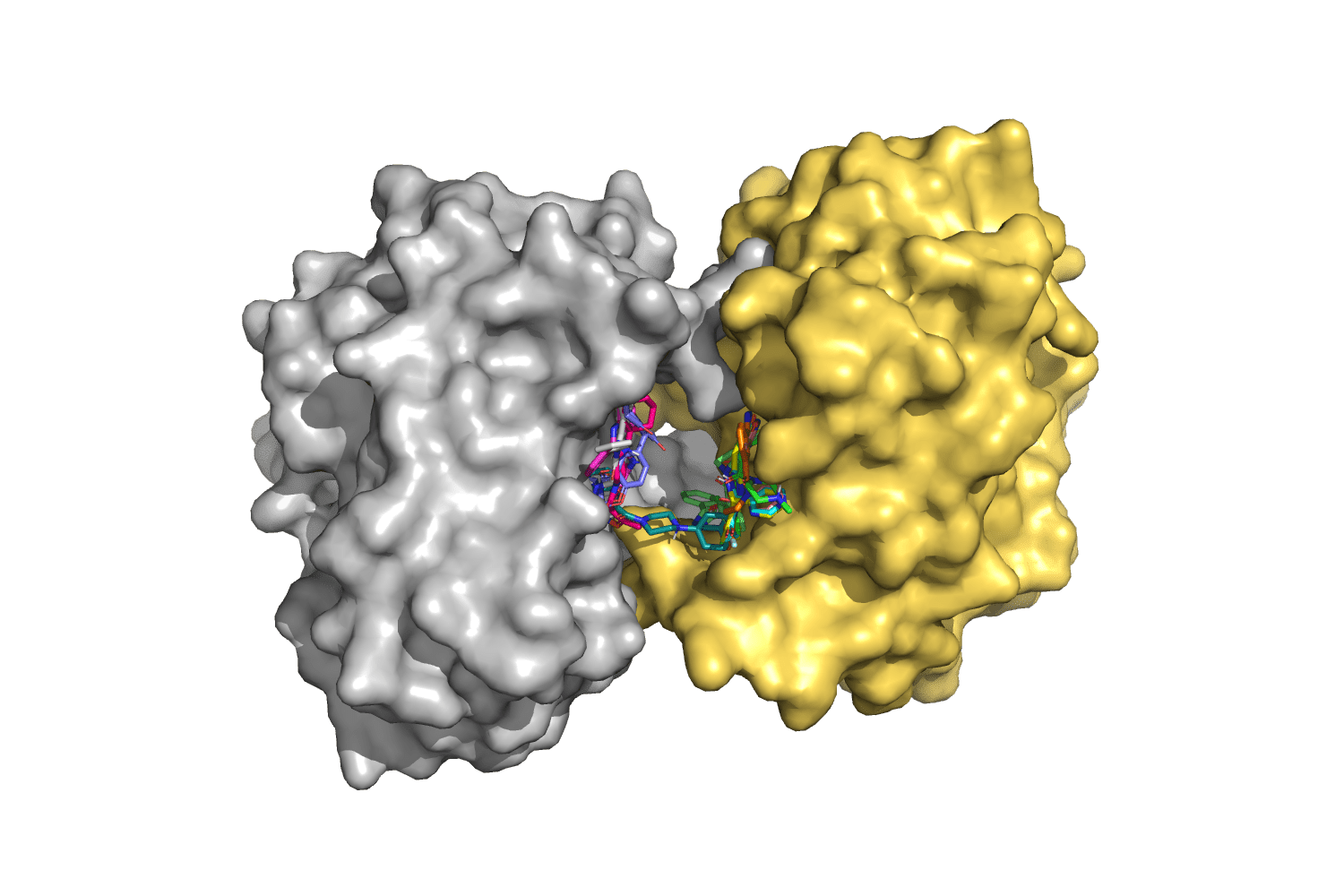
Figure 1: MMACHC protein dimers targeted with small molecules selected from a virtual library
We found that, compared to the wild-type protein, the mutants weakly bind vitamin B12 and do not efficiently promote its conversion. In collaboration with Maria Grazia Ortore (Università Politecnica delle Marche, Italy) and Heinz Amenitsch (Graz University of Technology, Austria), we also observed structural issues: these labile mutants easily lose the correct conformation and do not form dimers. This "biophysical" characterization of the proteins is a fundamental step in order to understand which are the chemical-physical features, i.e., what kind of defect, therefore, should be corrected to ensure the restoration of the protein functionality. This is particularly true for the study of the protein's spatial three-dimensional shape which is directly related to the protein function. All these data hint at a mutation-dependent destabilization of the protein architecture. Mutated MMACHC proteins adopt a suboptimal conformation which hinders its activity.
These structural defects can be countered by small molecules. Exploiting computational methods, we virtually tested 30’000 compounds and selected 11 predicted to bind MMACHC protein. The molecules were purchased and are currently being tested with the isolated proteins. Very preliminary data suggest that at least two of these candidates are able to bind the protein but we still need to understand if any of these molecules can restore functionality in protein mutants resulting from the CblC pathological mutations.
We are doing our best to provide at the end of the project indications on new molecules and/or new therapeutic strategies for CblC treatments”.

Figure 2: Research team involved in the project at IBF CNR in Palermo

Figure 2: Figure 3: Research team involved in the project at IBF CNR in Milano
