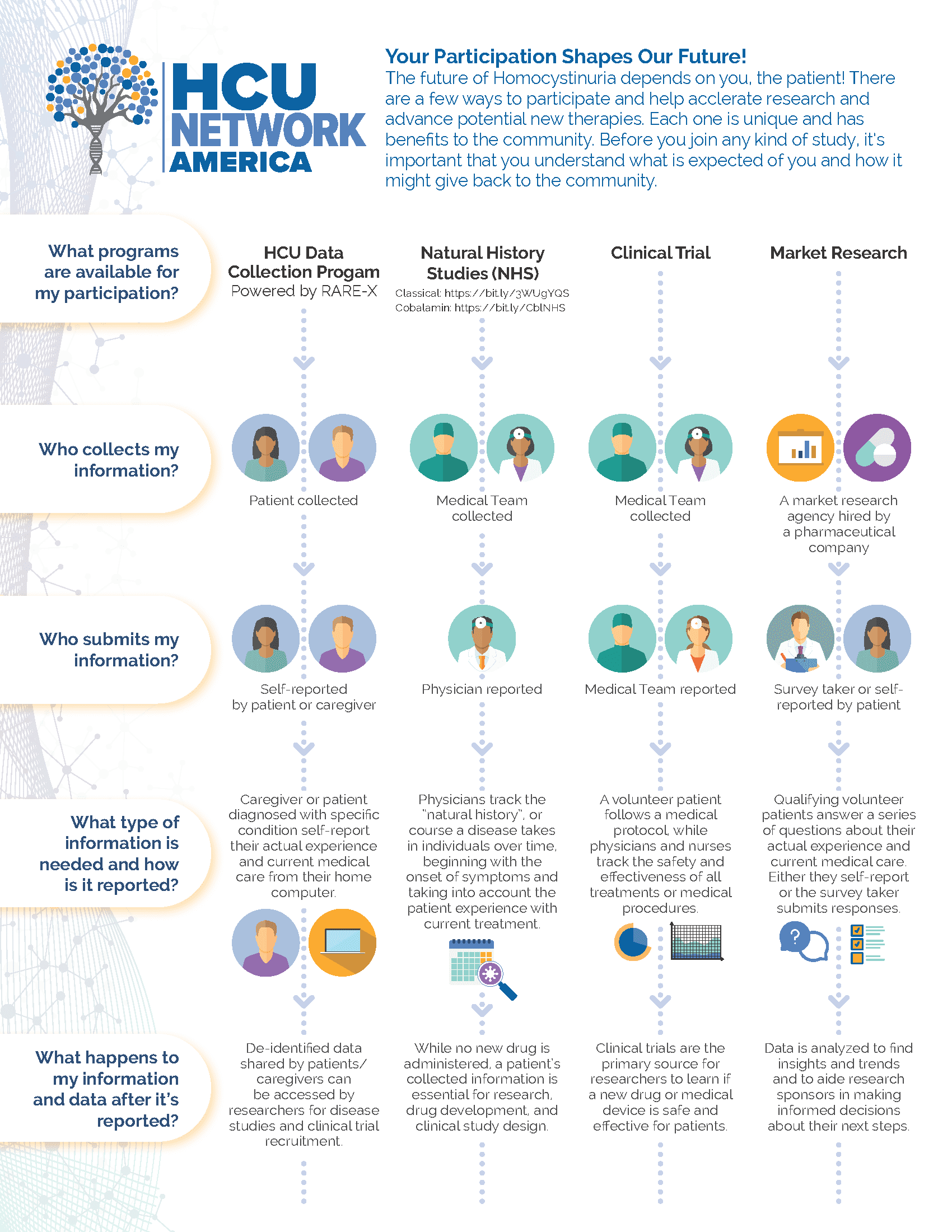
Externally led patient-focused drug development (EL-PFDD) meetings bring together patients and care partners, US Food and Drug Administration (FDA) representatives, pharmaceutical companies, doctors who are experts in the particular disease, and other stakeholders. For the meeting on Classical HCU, the goal is to hear from patients on what it's like to live with the condition so that we can better understand the patient experience. This information can help the FDA make informed decisions on approvals of potential medicines for Classical HCU and pharmaceutical companies to design meaningful clinical trials for patients.